A particular brand of HIV screening kit was recalled by the Health Sciences Authority as it gave users false negative results.
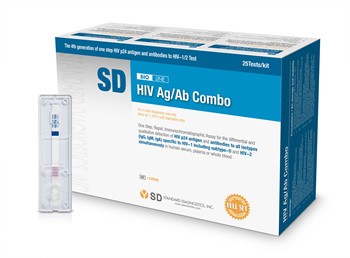
The SD Bioline HIV Ag/Ab Combo kits were supplied to 26 clinics and the National University Hospital.
According to reports, some users in the early stages of an HIV infection could receive an inaccurate false negative result.
The local importer, Unison Collaborator issued an urgent notice to the clinics and hospital to stop using the kits and retest patients who were tested negative using the affected product.
Each kit can be used for 25 tests, which means that up to 13,675 tests could have been carried out. HSA has clarified that some patients have returned for the retest.
All high-risk patients who use any brand of rapid tests kits have also been advised by HSA to return for another test to confirm their negative status.
HSA also highlighted that all rapid HIV tests are not meant to confirm an infection , but used only as an initial screening test. Test results that yield a positive must still be confirmed by sending a blood specimen to a clinical laboratory for Enzyme Immunoasay (EIA) testing and further confirmatory Western Blot testing if necessary.