The Health Sciences Authority (HSA) is alerting members of the public not to purchase or use the following health products:
-
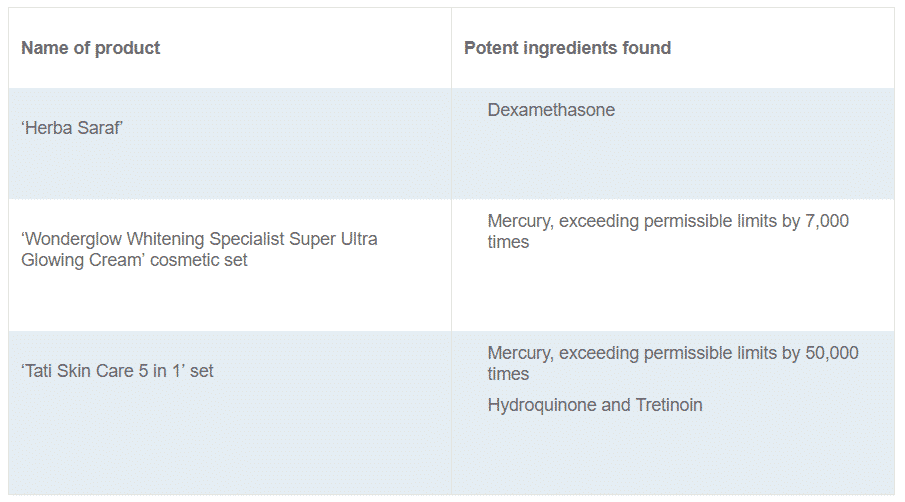
Herba Saraf.

-
Wonderglow Whitening Specialist Super Ultra Glowing Cream’ cosmetic set, which contains a ‘Day Cream’, a ‘Night Cream’ and a ‘Soap Bar’.

It was falsely labelled as “100% No Mercury Guaranteed”, as HSA found that the ‘Night Cream’ contained very high levels of mercury, exceeding the permissible limits by 7,000 times. -
Tati Skin Care 5 in 1’ cosmetic set 1, 2.
The products were obtained from Malaysia and sold on various online platforms.
HSA tested these products and found potent undeclared ingredients that are harmful and prohibited in these products:
Long-term exposure to cosmetic products with high levels of mercury can cause serious health consequences, including damage to the kidneys, and digestive and nervous systems. Inappropriate use of hydroquinone and tretinoin could cause adverse skin reactions such as redness, burning and peeling of the skin.
Advisory to consumers
Consumers are advised to take note of the following:
- See a doctor as soon as possible if you have taken ‘Herba Saraf’’. This product contains a potent steroid. Sudden discontinuation of steroids without proper medical supervision can cause serious withdrawal symptoms such as fatigue, confusion and low blood pressure.
- Stop using ‘Wonderglow Whitening Specialist Super Ultra Glowing Cream’ (‘Wonderglow’), and ‘Tati Skin Care 5 in 1’ cosmetic sets immediately as they contain very high levels of mercury and other undeclared potent ingredients that can lead to serious adverse reactions. See a doctor if you are experiencing adverse effects.
- Be cautious when purchasing health products online or from unfamiliar sources, even if they are recommended by friends or relatives. You cannot be certain what these products contain, and where and how they were made.
- Be wary of health products that promise quick and miraculous results or carry exaggerated claims like “100% Safe and Effective”. Such products may contain prescription medicines which should only be taken under medical supervision, or potent and prohibited ingredients that can harm you.
Adverse reaction that was reported to HSA
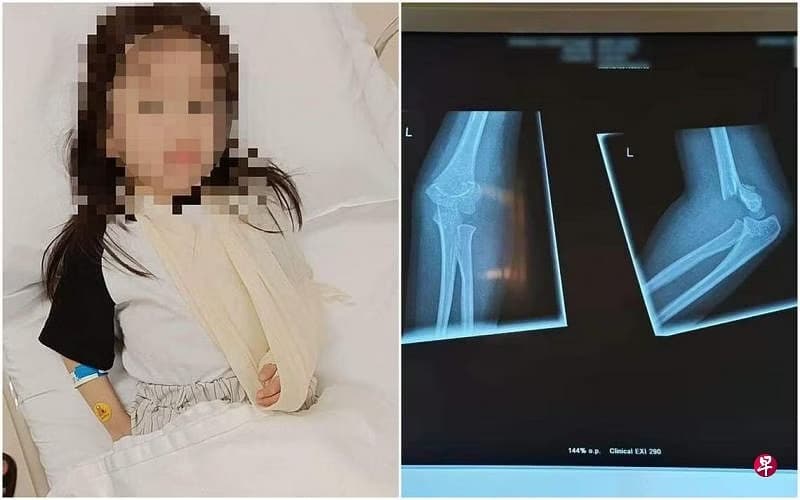
A consumer in her 40s had obtained ‘Herba Saraf’ from a relative in Malaysia who had bought it online. ‘Herba Saraf’ was labelled to contain herbal ingredients for the relief of pain such as joint pain and migraine. After taking it for more than a month for her knee pain, she was diagnosed with impaired glucose tolerance (poor blood sugar control).
Such a condition may increase one’s risk of diabetes and heart disease. She stopped taking the product immediately, suspecting that her condition was caused by it. The product was subsequently tested by HSA to contain dexamethasone, a potent steroid that is a prescription-only medicine that should only be used under medical supervision.
Inappropriate and prolonged use of steroids can result in Cushing’s syndrome, increased blood glucose levels leading to diabetes, high blood pressure, cataracts, muscular and bone disorders, and an increased risk of infections.
Importation and sale of illegal cosmetics
A woman in her 20s was detained at the Causeway for bringing the ‘Wonderglow’ and ‘Tati Skin Care 5 in 1’ cosmetic sets from Malaysia into Singapore. She had intended to sell these products online. HSA seized the products and tested them. HSA also found that ‘Wonderglow’ was being sold online by other sellers.
It was marketed as an anti-wrinkle and anti-aging product, with claims to brighten the skin in “as early as 3 days”. It was falsely labelled as “100% No Mercury Guaranteed”, as HSA found that the ‘Night Cream’ contained very high levels of mercury, exceeding the permissible limits by 7,000 times.
HSA had alerted the public to stop buying and using the ‘Tati Skin Care 5 in 1’ cosmetic set in June and September 20171,2. The product has resurfaced online, and recent tests showed that ‘Therapy Cream 1’ in the set contained mercury exceeding the permissible limits by close to 50,000 times.
‘Therapy Cream 2’ in the set was tested to contain hydroquinone and tretinoin. Both are potent western ingredients that should only be used under medical supervision.
Sellers and suppliers are warned that:
- The supply of ‘Herba Saraf’, ‘Night Cream’ in ‘Wonderglow’, ‘Therapy Cream 1’ and ‘Therapy Cream 2’ in ‘Tati Skin Care 5 in 1’ must be stopped immediately. These are illegal health products which contain undeclared potent ingredients.
- Anyone who supplies illegal health products is liable to prosecution and if convicted, may be imprisoned for up to 3 years and/or fined up to $100,000.
- HSA will not hesitate to take enforcement actions against sellers and suppliers who peddle illegal health products.
Members of the public who have any information on the sale and supply of these illegal products may contact HSA’s Enforcement Branch at Tel: 6866-3485 during office hours (Monday to Friday) or email: hsa_is@hsa.gov.sg.
If you’d like to contribute your story to us, drop us an email at editors@sureboh.sg and we’ll review it. We read each submission that comes to us within two weeks of receiving it.